Abstract
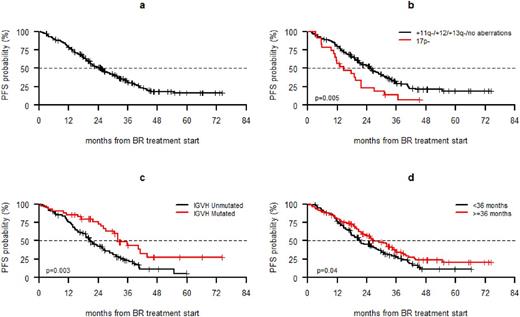
Introduction. In chronic lymphocytic leukemia (CLL), chemoimmunotherapy produced high overall response rates (ORR), prolonged progression free survival (PFS) and overall survival (OS). In the absence of a TP53 disruption, recent European guidelines recommend a chemoimmunotherapy regimen in first-line and in second line in patients who have attained a long PFS with the previous regimen. Uncertainty in the recommendations on first salvage treatment may partially derive from the consideration that the majority of studies have described efficacy data in an aggregate fashion including all relapsed/refractory setting. Since bendamustine and rituximab (BR) is one of most widely adopted second line regimen we performed a retrospective study within the GIMEMA-ERIC network to collect data on the efficacy and the safety of the BR regimen in second-line in a real-world setting. Comparison of the BR data with 114 Italian and UK non-trial patients treated with ibrutinib at first relapse will be presented at the meeting when additional follow-up is available for the ibrutinib-treated cohort.Methods . 237 CLL patients treated between January 2008 and December 2014 at 35 GIMEMA-ERIC centers were enrolled. Inclusion criteria were: i) CLL diagnosis according to the NCI-WG guidelines, ii) age ≥18 years, iii) one previous treatment with alkylating agents and/or purine analogues with or without monoclonal antibodies, iv) progression requiring second-line treatment according to the NCI criteria, v) at least 1 day of second-line treatment with BR (B 70mg/m2 days 1-2 and R 375 mg/m2 for the 1st course and 500 mg/m2 subsequently). The study was registered at ClinicalTrials.gov (NCT02491398). The primary endpoint was PFS at 12 months. The secondary endpoints were ORR, time to next antileukemic treatment (TTNT) and OS. Results. The median age was 70 years (range 39-87); 58.3% had ≥2 comorbidities, 46.9% a creatinine clearance ≤70 ml/min and 21.4% an advanced stage (i.e. Rai III-IV or Binet C). 73% had unmutated IGHV and 33.4% 11q- and/or 17p-. First-line treatment was chemoimmunotherapy in 59% of the cases; the remaining patients received chemotherapy. 18 patients (9,4%) were refractory to first line treatment. Treatment was discontinued in 72 patients (30.4%) because of toxicity (n=39), withdrawal of consent (n=7), progressive disease (n= 6) or other reasons (n=20). Treatment delay occurred in 22.5% of cases. 95 patients (40.4%) received 6 cycles without dose reduction. The PFS was 78.6%, 30.9% and 16.2% at 12, 30 and 60 months, respectively (Fig. 1a). Median PFS was 25 months with a median follow-up of 37.1 months. 17p- (p=0.0004; Fig. 1b), unmutated IGHV (p=0.0299; Fig. 1c) and advanced stage (p=0.0192) were associated with a shorter PFS at multivariate analysis, while a <36 month interval between first and second line treatment was associated with shorter PFS only at univariate analysis (p=0.04). The ORR was 82.3% and the probability of attaining a response was significantly lower in patients with 17p- (p=0.04). The TTNT at 12 months was 18.1%. A shorter TTNT was associated with 17p- (p=0.0215) and with chemoimmunotherapy (p=0.004) as first line regimen. First line treatment with chemoimmunotherapy was the only predictive factor at multivariate analysis (p=0.0096). 73 patients died due to CLL (n=14), infection with or without active CLL (n=27), second primary tumors (n=7); Richter's syndrome (n=4), other causes (n=21). OS at 12 months was 92.7%. Advanced stage (p=0.0276) and resistant disease (p= 0.0001) were the only adverse prognostic parameters at multivariate analysis. At 12 months, PFS and OS for patients without 17p- were 81.0% and 92.4%, respectively. 33% of the patients reported at least one grade 3-4 adverse event. Grade 3-4 neutropenia (including febrile neutropenia) occurred in 20.7% of cases, thrombocytopenia in 6 patients (2.5%), anemia in 3 patients (1.2%), 2 of whom had autoimmune hemolytic anemia. Grade 3-5 infections were recorded in 16 patients, 8 of whom had a lung infection. One case of fatal infection was reported (encephalitis). Conclusions. The data presented show that BR is an efficacious first salvage regimen in CLL except for those patients with unfavorable genetic features i.e. 17p- and/or unmutated IGHV and advanced stage. These data may assist clinicians in the selection of the most appropriate first salvage treatment in CLL.
Cuneo: Gilead: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; Abbvie: Honoraria, Other: Advisory Board; Roche: Honoraria, Other: Advisory Board. Rigolin: Celgene: Honoraria, Other: Advisory Board; Mundipharma: Other: Advisory Board; Gilead: Honoraria. Moreno: Janssen: Consultancy; Gilead: Consultancy, Research Funding. Galieni: Takeda: Other: Advisory Board; Abbvie: Other: Advisory Board. Laurenti: Roche: Other: Advisory Board; Gilead: Other: Advisory Board; Janssen: Other: Advisory Board; Abbvie: Other: Advisory Board. Molica: Roche: Other: Advisory Board; Jansen: Other: Advisory Board; AbbVie: Other: Advisory Board; Gilead: Other: Advisory Board. Montillo: Gilead Sciences, Inc.: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; Roche: Research Funding; Novartis: Honoraria; Genetech: Research Funding. Coscia: Abbvie: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Orlandi: Novartis: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Incyte: Speakers Bureau. Re: Gilead: Membership on an entity's Board of Directors or advisory committees. Gaidano: Amgen: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria. Foa: Roche: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Sandoz: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau. Follows: Roche: Other: advisory board and lecturing; Janssen: Other: advisory board and lecturing; Abbvie: Other: advisory board and lecturing; Gilead: Other: advisory board and lecturing. Ghia: AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal